Discovery in Clinical Research
Evidence-based medicine (EBM) has standardized care mainly through guidelines based on randomized controlled trials (RCTs), which effectively minimize confounding factors. However, RCTs have limitations, including limited generalizability due to strict eligibility criteria, delayed applicability due to long execution time, and ambiguous outcomes from composite endpoints. Registry-based observational studies address these issues by reflecting real-world practice. Registries help capture rare conditions and can serve as platforms for registry-based RCTs1. Ideally, clinical data should be collected electronically in real time and stored sequentially, though this has traditionally been considered resource-intensive.
Current Use of Electronic Medical Record (EMR) Data in Japan
Advances in information technology have led to the widespread implementation of hospital information systems (HIS) in Japan, enabling the collection of electric medical records (EMRs) and clinical test data. For instance, ischemic cardiovascular diseases—such as myocardial infarction and stroke—are major causes of morbidity in Japan. In myocardial infarction, risk factors are identified early through laboratory tests, and disease-specific data such as ECG, echocardiography, and catheterization accumulate over time. Treatment data include medications for risk factors, PCI procedures, and secondary prevention. These multimodal data clearly affect outcomes such as recurrence, heart failure hospitalization, and mortality. Collecting and integrating such data electronically holds promise for patient-level risk prediction and evaluation of treatment strategies through registry-based research platforms.
CLIDAS, Clinical Deep Data Accumulation System
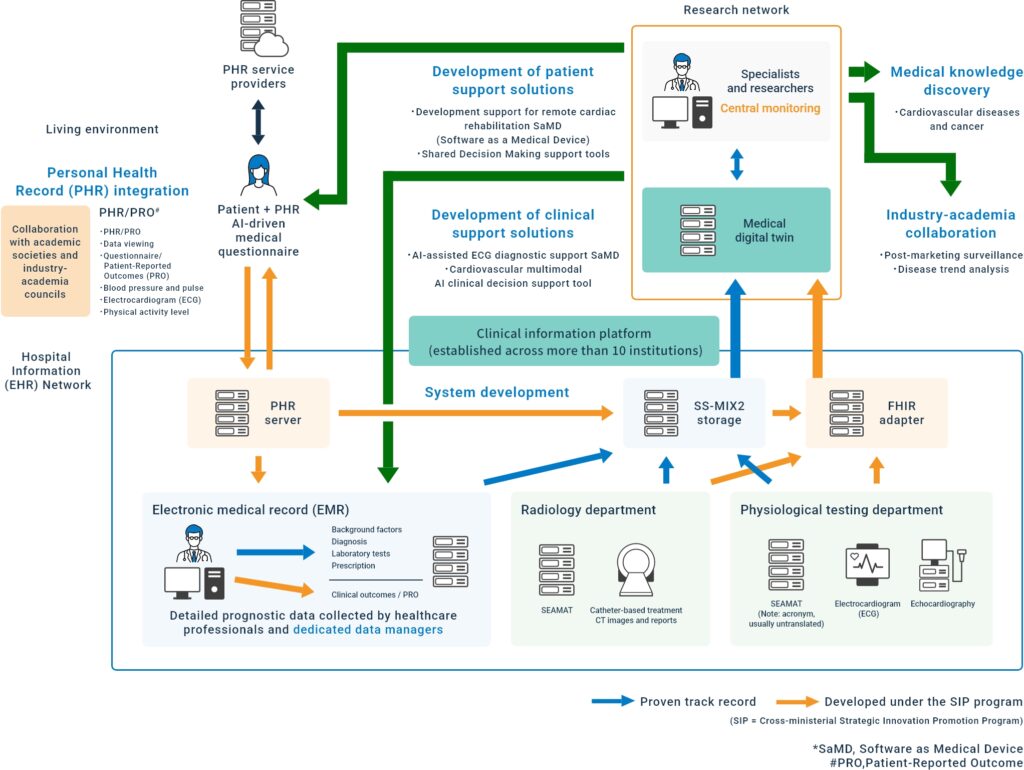
As a means to electronically extract multimodal clinical data from hospital information systems (HIS), the data storage area based on the standardized structured medical information exchange (SS-MIX) format—developed during the FIRST Program (2009–2013, led by Prof. Ryozo Nagai at the University of Tokyo) and later adopted as a national standard by Japan’s Ministry of Health, Labour and Welfare—is available for use. The current SS-MIX2 specification was established in 2012, and by 2024, more than 1,000 hospitals in Japan have implemented SS-MIX storages.
Supported by the Japanese Circulation Society (JCS) and the ImPACT Program (2015–2018, Project Leader: Prof. Ryozo Nagai, Jichi Medical University), the Clinical Deep Data Accumulation System (CLIDAS) research group developed the J-IMPACT system (Japan Ischemic Heart Disease Multimodal Prospective Data Acquisition for Precision Treatment)2. This system enables the collection of patient demographics, prescription data, and laboratory test results from SS-MIX2 standard storage, as well as physiological test data, cardiac catheterization reports (CAIRS)3, and PCI treatment records from SS-MIX2 extended storage via intrahospital networks. The CLIDAS research group expanded across multiple institutions (see figure below).
UMIN Clinical Trial Registry ID UMIN000057338
Patient outcome events are critical in all clinical research, and the CLIDAS Research Group has trained data managers to capture these events, especially in observational studies. Based on the analysis of the medical digital twin, the group is working on medical knowledge discovery, industry-academia collaboration, and the development of patient and medical support solutions.
SEAMAT
Since the CLIDAS system is based on the accumulation of multimodality medical data into SS-MIX2 storage, standardization of data formats for each modality is extremely important for deployment at multiple facilities. The IT/Database committee of the Japanese Society of Cardiology (JCS) tarted to develop the Standard Export datA forMAT (SEAMAT)4 in 2014 to promote the utilization of cardiovascular-related test data in electronic medical records, and the IHE-J Cardiology Committee has published the SEAMAT standard and specifications for storing data in SS-MIX2 extended storage on the JCS website since 2016 (http://www.j-circ.or.jp/itdata/jcs_standard.htm).
As of 2025, standardized formats for electrocardiograms (ECG), echocardiography, cardiac catheterization/PCI reports, cardiac nuclear medicine, and coronary CT report data have been published, and efforts are being made to promote compliance with SEAMAT among equipment manufacturers. It is expected that adoption will further advance as healthcare institutions, the end-users, become more aware of SEAMAT and its advantages.
Current Status and Future Prospects
Since 2023, with the support of the Cabinet Office’s Strategic Innovation Creation Program (SIP), the number of participating institutions in CLIDAS has expanded to 13. At these institutions, multiple vendors’ EMR systems, cardiac catheterization reporting systems, and physiological examination systems are operational. Validation of CLIDAS is being conducted through data collection based on the SEAMAT standards.
CLIDAS enables a multi-institutional collection of deep data on cardiovascular care and patient outcomes across multiple modalities, which will allow clinical questions to be examined based on up-to-date real-world data, even amid constantly evolving pharmacotherapy and cardiovascular interventions. Furthermore, it is expected to serve as a clinical information platform that supports industry-academia collaboration and the research and development of new medical technologies.
Support from academic societies and public research fundings

Clinical Effectiveness Database Project in the Japanese Circulation Society

Health and Labour Sciences Research Grant

Cross-ministerial Strategic Innovation Promotion Program (SIP)
References
- James S., Rao S. V, Granger C.B.: Registry-based randomized clinical trials–a new clinical trial paradigm. Nat. Rev. Cardiol., 12:312–316, 2015. ↩︎
- Matoba T, Kohro T, Fujita H, Nakayama M, Kiyosue A, Miyamoto Y, Nishimura K, Hashimoto H, Antoku Y, Nakashima N, Ohe K, Ogawa H, Tsutsui H, Nagai R. Architecture of the Japan Ischemic Heart Disease Multimodal Prospective Data Acquisition for Precision Treatment (J-IMPACT) System. Int Heart J. 2019;60:264-270. ↩︎
- Kohro T, Iwata H, Fujiu K, Manabe I, Fujita H, Haraguchi G, Morino Y, Oguri A, Ikenouchi H, Kurabayashi M, Ikari Y, Isobe M, Ohe K, Nagai R. Development and Implementation of an Advanced Coronary Angiography and Intervention Database System. Int Heart J. 2012;53:35-42. Int. Heart J., 53:35–42, 2012. ↩︎
- Nakayama M, Takehana K, Kohro T, Matoba T, Tsutsui H, Nagai R. Standard Export Data Format for Extension Storage of Standardized Structured Medical Information Exchange. Circ Rep. 2020;2:587-616. ↩︎
