Industry-Academia Collaboration
Industry-academia collaboration refers to cooperative efforts between companies (industry) and universities or research institutions (academia) aimed at developing new technologies and applying research findings in practical settings. In the medical field, such collaborations are driving the development of innovative diagnostic and therapeutic technologies, as well as improving the quality of healthcare through data utilization.
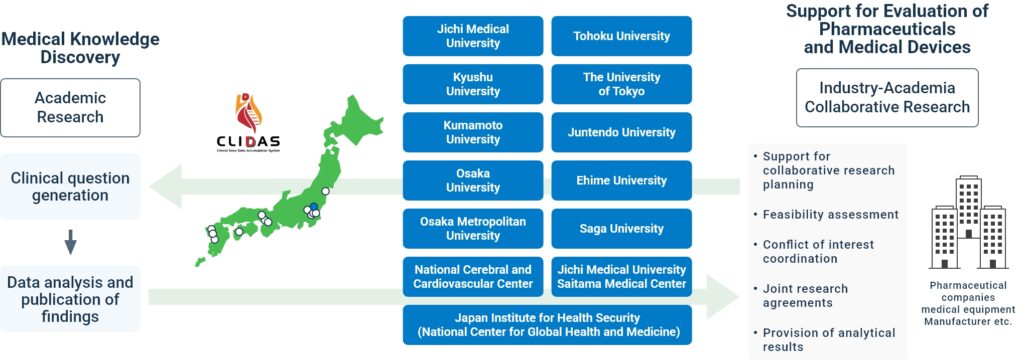
Academic Research and Industry-Academia Collaborative Research in Knowledge Discovery and Translational Innovation
Our research activities are structured as follows:
CLIDAS Clinical Datasets
The platform includes standardized data from electronic medical records, examination systems, outcome data collected by dedicated data managers, and analyses conducted by physician researchers.
01. CLIDAS-PCI
A retrospective, opt-out dataset of approx. 10,000 patients undergoing percutaneous coronary intervention (PCI), including comorbidities, PCI reports, longitudinal laboratory tests and prescriptions, ECGs and echocardiograms, coronary CT images and reports, and clinical outcomes from chart review.
02. CLIDAS-HF/HTN
A retrospective, opt-out dataset of approx. 25,000 heart failure patients and 20,000 hypertension patients, containing background and comorbidities, longitudinal laboratory tests and prescriptions, ECGs and echocardiograms, and clinical outcomes from chart review.
03. CLIDAS-DPC
A dataset of approx. 100,000 cases with Diagnosis Procedure Combination (DPC) insurance claim data and short-term clinical outcomes.
04. Multimodal AI Training Data
A datasets for artificial intelligence (AI) training collected from CLIDAS-affiliated institutions, including approx. 1 million chest X-rays and 3 million ECGs, for use in medical AI research and development.
Industry-Academia Collaboration Workflow
- Support for designing joint research plans based on clinical questions
- Feasibility assessment (*optional)
- Formulation of the joint research protocol
- Preparation of a research budget estimate
- Execution of a bilateral joint research agreement with the lead institution
- Data analysis and delivery of analytical results
- Academic presentations and publications co-authored with the collaborating company
Examples of CLIDAS Database Utilization
- Investigation of real-world prescription patterns, including associations with clinical outcomes
- Post-marketing surveillance of drugs (e.g., effectiveness, adverse events)
- Patient recruitment support for clinical trials
- Identification of drug targets and biomarkers by linking medical data with biospecimens
- Other uses upon consultation
